
Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4676
Research Article(ISSN: 2637-4676) 
Determination of Genetic Variations of Post-Micropropagated Sweet Orange (Citrus Sinensis (L.) Osbeck) micro-shoots by ISSR Marker Technique Volume 10 - Issue 2
Sevinc Guldag1,2, Damla Ekin Ozkaya1,3, Ergun Kaya1*
- 1Molecular Biology and Genetics Department, Faculty of Science, Mugla Sitki Kocman University, Kotekli 48000, Mugla, Turkey
- 2Faculty of Engineering, Department of Bioengineering, Marmara University, Kadikoy-34722 Istanbul, Turkey
- 3Pathology Laboratory Techniques Program, School of Advanced Vocational Studies, Dogus University, Umraniye 34775, Istanbul, Turkey
Received: January 11, 2023; Published: January 18, 2023
Corresponding author: Ergun Kaya, Molecular Biology and Genetics Department, Faculty of Science, Mugla Sitki Kocman University, Turkey
DOI: 10.32474/CIACR.2023.10.000336
Abstract
The environmental conditions that the plant is exposed to during micropropagation may cause some variations in the species, which may cause the loss of some properties of the plant and even decrease its economic value. In this study, clonal propagation was carried out by four-week subculturing procedures of rootstocks obtained by using seeds of traditional citrus species, Citrus sinensis (L.) Osbeck (sweet orange) as starting material. Then, the genetic stability of the clones obtained from the same rootstock plant was determined by using ISSR primers, one of the molecular marker techniques. When the visualization of the PCR products were analyzed, the genetic stability was calculated as 92%, 94.29% and 96.57% at the end of one year in micro-shoots developed from rootstocks of a total of three C. sinensis clones. This ratio is considered as successful results in determining the maintainability of genetic stability.
Keywords: Citrus sinensis; genetic stability; ISSR PCR; micropropagation; somaclonal variation
Introduction and Background
In the world, the citrus trees are one of the most planted fruit species. These fruit trees are of excellent importance as production is higher than other economically important trees and is crucial for the implementation of preservation strategies and the prospective use of genetic resources [1]. There is an increasing demand and consumption of oranges in direct proportion to its widespread use and the increasing population rate around the world. While a total of 98 million tons of citrus was cultured worldwide in the 2020/21 production season, there is a 5% increase in total citrus production compared to the previous time. Orange, on the other hand, provides 51% of the total citrus production, followed by grapefruit with 7%, lemon with 9%, and tangerine with 34% respectively. Compared to the previous season, the highest increase in production among citrus products was seen in orange, with a rate of 8%. While Brazil maintains its leadership in world orange production with a production share of 16.9 million tons, Turkey ranks seventh by meeting 3% of the world’s orange production [2]. Plant micropropagation techniques has emerged as a effective tool for the development and production of different woody species, including Citrus [3,4]. Micropropagation from common plant tissue cultures is also an important technique for citrus because it ensures maximum genetic stability of the resulting plants. Therefore, micropropagation becomes important and remains the only viable alternative to build up a stock of valuable plant material. There are different strategies routinely used for micropropagation of citrus [5,6]. Among them, axillary augmentation using the nodal segment as explant is considered the best as it does not contain a callus phase, thus minimizing the risk of somaclonal variation and economically offering optimum proliferation rate. Cost-effective and fast in vitro propagation procedures of plant species of interest with these techniques will be of great interest in this context to increase mass reproduction and preserve germplasm stocks for many years [7-9].
To achieve crucial development in the cultivars of C. sinensis, there is an immediate necessity to preserve the main characteristics of cultures and/or varieties and to determine and investigate existing genotypes. The molecular, biochemical, morphological and cytological alterations can be detected in in vitro cultures. Of these, DNA based marker techniques are traditional beneficial tools used to characterize and confirm the genetic fidelity of in vitro grown plant species. The use of DNA based marker systems has been a advantageous and appropriate strategy to determine wild-types and cultivars of Citrus species and to evaluate the genetic diversity of them. DNA based marker systems such as RFLP, AFLP, SSR, ISSR, RAPD, and other different markers have been employed for germplasm identification, systematics and phylogenetic analysis, and genetic diversity studies [10-14]. The present study aimed to detect a possible genetic variation during the clonal propagation of C. sinensis rootstocks using the ISSR marker technique by in vitro tissue culture method. Thus, an innovative approach will be developed for the production of genetically stable seedlings in micro-shoot rootstock production by year-round clonal propagation under aseptic conditions with biotechnological methods.
Material and Methods
Micropropagation Applications
The plant material was obtained from Muğla Metropolitan Municipality, Directorate of Agricultural Services, Muğla Local Seed Bank collection (Turkey). These seeds were surface sterilized by the sterilization protocol modified by Kaya et al. [8] for citrus cultivars. In the method, the seeds were treated with 70% ethanol for 5 minutes, 10% H2O2 for 5 minutes and twice with 20% commercial bleach for 10 minutes and then washed with sterile distilled water until completely rinsed [15]. After the seeds were dried in a laminar flow cabinet for 10 minutes, the testa was removed and the seeds were then cultured in solid MS [16] supplemented with 20 gL-1 sucrose, 1 mgL-1 6-Benzylaminopurine, and 7 gL-1 agar (pH 5.8) under standard growth conditions (27±2 °C temperature, 16/8 h photoperiod and 50 μmol-1m-2s-1 white daylight fluorescent lamp). Micro-shoots germinated from each seed were subcultured separately and propagated clonally for 1 year with a subculture period of 4 weeks.
Molecular Analyzes
DNA was isolated from micro-shoots of the three clones (CS1, CS2, and CS3) derived from three different seeds grown on the same optimal medium [8] for one year and the subcultured regularly every month. DNA isolation was realized with the CTAB method developed by Doyle and Doyle [17]. PCR analyzes were performed for the genetic stability by selecting 10 of the 24 different ISSR primers that gave the best band profile. PCR was performed with a 25 μL reaction mix containing 40 ng of DNA, 0.4 mM primer, 0.4 mM of each dNTP, 2.5 mM MgCl2, and 1 unit of Ampliqon Taq DNA Polymerase. PCR cycles were set by denaturation at 95 °C for 3 minutes, followed by 35 cycles of 95 °C for 15 seconds, 54 °C for 30 seconds, and 68 °C for 3 minutes and then a 10-minute extension phase at 72°C was performed [18]. The PCR products were separated on a 1.5% agarose gel at 80 V and visualized under UV light by staining with 0,5 μg ml-1 ethidium bromide solution. Band profiles were scored as 1 (present) or 0 (absent) and all data were analyzed to determine genetic stability [18,19]. POPGENE Version 1.32 program was used to determine genetic stability.
Results and Discussion
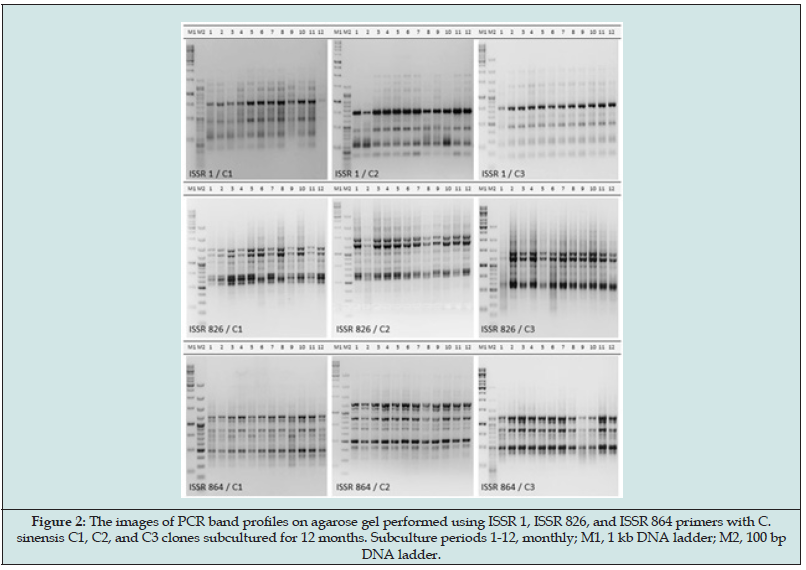
The micro-shoots of three different C. sinensis clones were successfully in vitro propagated by subculturing at regular intervals for 12 months. There was no morphological variation in samples that produced healthy stem and leafy micro-shoots on regeneration nutrient media during each four-week subculture period (Figure 1). By ISSR analysis of the samples obtained as a result of subculturing for one year within each clone, the bands that may occur later (from 1 to 0) or disappearing bands (from 0 to 1) were determined. A total of 175 loci were identified as a result of the analysis of 10 primers. Mostly monomorphic bands and very few polymorphic bands were observed (Figure 2). According to the analysis results obtained by the POPGENE Version 1.32 program used to determine the genetic stability, the average stability levels of 3 seeds at the end of 12 months were determined (Table 1). As a result of the results obtained, there was a very low variation of 8% in the genetic material of the CS1 individual, 5.71% in the genetic material of the CS2 individual and 3.43% in the genetic material of the CS3 individual. The determined genetic stability percentages were calculated as 92% for CS1, 94.29% for CS2 and 96.57 for CS3, respectively. DNA isolation of Citrus sinensis, which was taken from sweet orange samples and successfully developed in vitro from 3 different seeds, and then micropropagated provided that each seed was subcultured separately at regular intervals for 12 months, was successfully performed. Then, ISSR-PCR was performed to determine genetic stability. In the light of the data obtained from the imaging of the PCR products on the gel, the POPGENE Version 1.32 program used to determine the genetic stability determined the average stability levels at the end of 12 months of the clones developed from three individuals. As a result of the results obtained, low variations such as 8% in the genetic material of the CS1 clone, 5.71% in the genetic material of the CS2 clone and 3.43% in the genetic material of the CS3 clone were determined. The results can be considered successful as they cover a long period of time such as one year. Similarly, Azizi et al. [24] investigated the genetic stability using SSR primers after clonal propagation in a study on six varieties of sugarcane (Saccharum officinarum L.) and determined the similarity index to rootstocks of all varieties as 0.94 after 9 subcultures. On the other hand, in another study, Pendli et al. [25] determined the genetic stability using RAPD and ISSR primers in the protocol they developed for the genetically stable in vitro propagation of Solanum trilobatum L. and obtained genetically identical in vitro material with the rootstock.
Figure 2: The images of PCR band profiles on agarose gel performed using ISSR 1, ISSR 826, and ISSR 864 primers with C. sinensis C1, C2, and C3 clones subcultured for 12 months. Subculture periods 1-12, monthly; M1, 1 kb DNA ladder; M2, 100 bp DNA ladder.
In a study conducted by Ozudogru et al. [15] in the protocol developed for the effective micropropagation of Thymus vulgaris L. plant, the genetic stability of the clones subcultured for 10 months was analyzed with RAPD primers and the genetic stability was determined as 100%. In this context, some studies have confirmed that in vitro propagated plantlets maintain their genetic stability during cell division and differentiation under in vitro conditions [26,27]. However, it should be noted that there is still a risk of changes [28] induced by in vitro processes (eg, stress, auxin-cytokinin ratio, and nutritional conditions) [29]. While there are many studies in the literature reporting the frequency of genetic changes among micropropagated plants [28,30], there are also some studies showing the maintenance of genetic stability [31,32]. These changes are often not favored as they are heritable and can be continued in subsequent generations of micropropagated plantlets [33]. Although a large number of molecular markers are available for the detection of such changes at the DNA level, the ISSR technique is the most widely used, requiring little template DNA, no pre-sequence information, and producing data faster and easier than many other techniques. The method also provides the advantages and reliability of SSR technology without requiring prior sequence knowledge [19,34,35]. Somaclonal variations, defined as variations associated with in vitro regeneration of plants, are associated with an increase in the length of the subculture or the number of subcultures, and these factors increase the mutation frequency. For this reason, it is necessary to periodically renew the stock cultures. Maggon and Singh [36] observed that citrus sprout seeds originate mainly from callus formed on the cut surface of explants cultured in a BA-containing medium. This approach to shoot regeneration is associated with an increased risk of genetic instability [36]. Morphological markers have been widely used to identify somaclonal variants, although some genetic changes are not reflected in the observed phenotypic variation. In the same cases, the anomalies observed in non-species plants could only be noticed after many years of cultivation in field conditions. The economic impact of somaclonal variation in citrus fruits may be high as they have long life cycles.
Many citrus species bear fruit 2-3 years after transplanting, and investigating somaclonal variation on fruit morphological traits is time consuming and costly [37]. To achieve significant improvement in C. sinensis cultivars, there is an urgent need to preserve the essential characteristics of varieties/cultures and to characterize and evaluate existing genotypes. The use of molecular markers has been a valuable and appropriate strategy to identify Citrus species, cultivars and biotypes and to investigate the genetic diversity of citrus species. Molecular marker techniques such as RAPD, ISSR, RFLP, SSR, AFLP and other markers have been used for germplasm characterization, genetic diversity studies, systematics and phylogenetic analysis [10]. Among them, randomly amplified polymorphic DNA (RAPD) markers have been most widely used for the characterization of plant species [38, 39]. RAPD has attracted more attention due to the simplicity of the procedure, its low cost, and the very small amount of DNA required for analysis. In citrus, RAPD markers have been used for variety identification, genetic mapping, genetic diversity assessment and other breeding programs [40].
Concluding Remarks
In this study, with ISSR molecular markers; After micropropagation of C. sinensis, which was developed in vitro, in order to preserve its genetic stability without any change in its molecular structure, it was examined whether this purpose was achieved. Especially in plants transferred directly from nature to the culture medium, some changes may occur in the genetic material, which is generally called somaclonal variation, as a result of the change of abiotic and biotic factors in which the plant is found. It can reduce its economic value. If there is no change in the genetic structure of the plant material to be developed due to the medium or subculturing stages, or if it is experienced at a very low rate, the result is considered successful. Large deviations in the genetic stability of C. sinensis, which is an economically important and medicinal aromatic plant, means that the plant loses its unique properties and benefits. In this context, the 12-month period has been completed successfully, allowing for faster and pathogen-free reproduction of C. sinensis, which is aimed for micropropagation with a successful subculturing while maintaining its genetic stability, compared to its natural environment. Afterwards, some of the plantlets obtained were used for DNA isolation, while some of them were adapted to the soil with the climatization technique in order to bring them back to nature.
Acknowledgment
This study was supported by Mugla Sitki Kocman University, Scientific Research ProjectsCoordination Unit (Mugla, Turkey, MSKU-BAP, Project Number: 19/076/05/1/2).
References
- Castaneda Cardona CC, Portela Puerta R and Morillo Coronado Y (2021) Molecular characterization with ISSR markers of the citrus collection from the Universidad de los Llanos. Revista de Investigacion Agraria y Ambiental 12(2): 67-84.
- Statista 2022, FAO; USDA foreign agricultute servise 2021/2022.
- Mehbub H, Akter A, Akter MA, Mandal MSH and Hoque MA, et al. (2022) Tissue Culture in Ornamentals: Cultivation Factors, Propagation Techniques, and Its Application. Plants 11(23): 3208.
- Einset JW (1978) Citrus tissue culture: stimulation of fruit explant cultures with orange juice. Plant Physiology 62(6): 885-888.
- Chamandoosti F (2017) The utilities of Citrus tissue culture. International Journal of Environmental & Agriculture Research 3(9): 36-46.
- Paudyal KP and Haq N (2000) In vitro propagation of pummelo (Citrus grandis L. osbeck). In Vitro Cellular & Developmental Biology 36(6): 511-516.
- Lalhmachhuani H, Hazarika TK, Lalhriatpuia C, Premabati T and Singh TR (2021) In vitro propagation and antioxidant properties of hatkora (Citrus macroptera) from North-East India. Research on Crops 22(1): 87-95.
- Kaya E, Souza F, Gokdogan EY, Ceylan M, and Jenderek M (2017) Cryopreservation of citrus seed via dehydration followedby immersion in liquid nitrogen. Turkish Journal of Biology 41(1): 242-248.
- Ozkaya DE, Souza FVD and Kaya E (2022) Evaluation of Critical Points for Effective Cryopreservation of Four Different Citrus spp. Germplasm. Horticulturae 8(11): 995.
- Weising K, Nybom H, Pfenninger M, Wolff K and Kahl G (2005) DNA fingerprinting in plants: principles, methods, and applications, CRC press pp 31.
- Kaya E, Vatansever R and Filiz E (2018) Assessment of the genetic relationship of Turkish olives (Olea europaea subsp. europaea) cultivars based on cpDNA trnL F regions. Acta Botanica Croatica 77(1): 88-92.
- Gokturk RS, Dusen O, Kaya E, Gurcan B and Sarpkaya U (2019) A new variety of Plocama calabrica (Rubiaceae) from Denizli (Turkey) onfirmed by morphological and molecular ISSR markers. Acta Botanica Croatica 78(2): 142-146.
- Dusen O, Gokturk RS, Kaya E, Sarpkaya U and Gurcan B (2018) Morphological and molecular determination of a new Viola species (Violaceae) from Turkey. Phytotaxa 369(1): 37-46.
- Ustuner H, Yavuz M, Gokturk RS, Kaya E and Galatalı S (2022) Morphological and molecular notes of Gypsophila pilulifera. Phytotaxa 556(2): 99-118.
- Ozudogru EA, Kaya E, Kirdok E and Issever Ozturk S (2011) In vitro propagation from young and mature explants of thyme (Thymus vulgaris and T. longicaulis) resulting in genetically stable shoots. In Vitro Cellular & Developmental Biology Plant 47(2):309–320.
- Murashige T and Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia plantarum 15(3): 473-497.
- Doyle JJ and Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Focus 19(1): 11-15.
- Kaya E (2015) ISSR analysis for determination of genetic diversity and relationship in eight Turkish olive (Olea europaea L.) cultivars. Notulae Botanicae Horti Agrobotanici Cluj Napoca 43(1): 96-99.
- Kaya E and Souza FVD (2017) Comparison of two PVS2-based procedures for cryopreservation of commercial sugarcane (Saccharum spp.) germplasm and confirmation of genetic stability after cryopreservation using ISSR markers. In Vitro Cellular & Developmental Biology Plant 53(4): 410-417.
- Pakseresht F, Talebi R and Karami E (2013) Comparative assessment of ISSR, DAMD and SCoT markers for evaluation of genetic diversity and conservation of landrace chickpea (Cicer arietinum L.) genotypes collected from north-west of Iran. Physiology and Molecular Biology of Plants 19(4): 563-574.
- Felix FC, das Chagas KPT, dos Santos Ferrari C, de Almeida Vieira F and Pacheco MV (2020) Applications of ISSR markers in studies of genetic diversity of Pityrocarpa moniliformis. Revista Caatinga 33(4): 1017-1024.
- Yuan CY, Wang P, Chen PP, Xiao WJ and Zhang C, et al. (2015) Genetic diversity revealed by morphological traits and ISSR markers in 48 Okras (Abelmoschus escullentus L.). Physiology and Molecular Biology of Plants 21(3): 359-364.
- Gwinner R, Setotaw TA, Rodrigues FA, França DVC and da Silveira FA, et al. (2016) Population structure of Annona crassiflora: an endemic plant species of the Brazilian Cerrado. Genetics and Molecular Research 15(4): gmr15049137.
- Azizi AAA, Roostika I, Reflinur R and Efendi D (2020) Analysis of Genetic Stability of Micropropagated Sugarcane in Different Subculture Frequencies Using SSR Marker. Jurnal Penelitian Tanaman Industri 26(1): 49-57.
- Pendli S, Rohela GK, Jogam P, Bylla P, Korra R and Thammidala C, et al. (2019) High frequency in vitro plantlet regeneration in Solanum trilobatum L., an important ethno-medicinal plant and confirmation of genetic fidelity of R 1 plantlets by using ISSR and RAPD markers. Vegetos 32(4): 508-520.
- Ostry M, Hackett W, Michler C, Serres R and McCown B (1994) Influence of regeneration method and tissue source on the frequency of somatic variation in Populus to infection by Septoria musiva. Plant Science 97(2): 209-215.
- Shenoy VB and Vasil IK (1992) Biochemical and molecular analysis of plants derived from embryogenic tissue cultures of napier grass (Pennisetum purpureum K. Schum). Theoretical and Applied Genetics 83(8): 947-955.
- Rani V, Parida A and Raina SN (1995) Random amplified polymorphic DNA (RAPD) markers for genetic analysis in micropropagated plants of Populus deltoides Marsh. Plant Cell Reports 14(7): 459-462.
- Modgil M, Mahajan K, Chakrabarti SK, Sharma DR and Sobti RC (2005) Molecular analysis of genetic stability in micropropagated apple rootstock MM106. Scientia Horticulturae 104(2): 151-160.
- Gimenez C, De Garcia E, De Enrech NX and Blanca I (2001) Somaclonal variation in banana: cytogenetic and molecular characterization of the somaclonal variant CIEN BTA-03. In Vitro Cellular & Developmental Biology Plant 37(2): 217-222.
- Rout GR, Das P, Goel S and Raina SN (1998) Determination of genetic stability of micropropagated plants of ginger using random amplified polymorphic DNA (RAPD) markers. Botanical Bulletin of Academia Sinica 39(1): 23-27.
- Javouhey M, Daguin F and Letouze R (1999) Somatic embryogenesis, an efficient tool for date palm (Phoenix dactylifera L.) industrial micropropagation. Characterization and genetic stability of original offshoots and regenerated plantlets by RAPD markers. Acta Horticulturae 530: 237-242.
- Breiman A, Rotem Abarbanell D, Karp A and Shaskin H (1987) Heritable somaclonal variation in wild barley (Hordeum spontaneum). Theoretical and Applied Genetics 74(1): 104-112.
- Reddy MP, Sarla N and Siddiq EA (2002) Inter simple sequence repeat (ISSR) polymorphism and its application in plant breeding. Euphytica 128(1): 9-17.
- Vijayan K (2005) Inter simple sequence repeat (ISSR) polymorphism and its application in mulberry genome analysis. International Journal of Industrial Entomology 10(2): 79-86.
- Maggon R and Singh BD (1995) Promotion of adventitious bud regeneration by ABA in combination with BAP in epicotyl and hypocotyl explants of sweet orange (Citrus sinensis L. Osbeck). Scientia Horticulturae 63(1-2): 123-128.
- Carimi F and De Pasquale F (2003) Micropropagation of citrus. In Micropropagation of woody trees and fruits Springer pp 589-619.
- Williams JG, Kubelik AR, Livak KJ, Rafalski JA and Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic acids research 18(22): 6531-6535.
- Kaya E, Balcı MA, Akguller O, Galatalı S and Yeniocak S, et al. (2021) Development of an optimum proliferation medium via the graph kernel statistical analysis method for genetically stable in vitro propagation of endemic Thymus cilicicus (Turkey). Acta Botanica Croatica 80(2): 199-207.
- Das A, Sarkar J, Mondal B and Chaudhuri S (2004) Genetic diversity analysis of Citrus cultivars and rootstocks of the north eastern India by RAPD markers. The Indian Journal of Genetics and Plant Breeding 64(4): 281-285.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...






